Patients OverviewOur purpose is patients
Our vision is to provide life-long cures to patients suffering from serious diseases. But we cannot do it alone. We are committed to working with and learning from the patient community to help support patients and their loved ones.
We believe in making fearless, intentional choices that make a positive impact on patients’ lives. We aim to do this by:
- listening to and integrating patient and caregiver perspectives throughout Beam
- supporting and collaborating with patient advocacy groups and community-based organizations to meet unmet needs
- respecting the independence of the organizations and privacy of the patients we serve
For more information, contact [email protected].
Understanding Base Editing
Curing serious diseases one letter at a time
Spelling matters
The genetic information that makes you you is stored as a unique code in your DNA. The code is written with just four chemical bases, or letters, labeled A, C, G, and T. People have 3 billion of these letters in their DNA. The letters are arranged in a specific sequence that can spell the difference between health and disease.
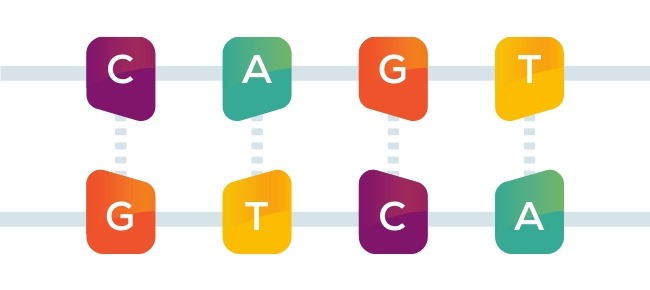
A single spelling error in the code—known as a point mutation—can lead to serious illness just as a single misspelled letter in a word can lead to an entirely different meaning: list or lost, batter or better.
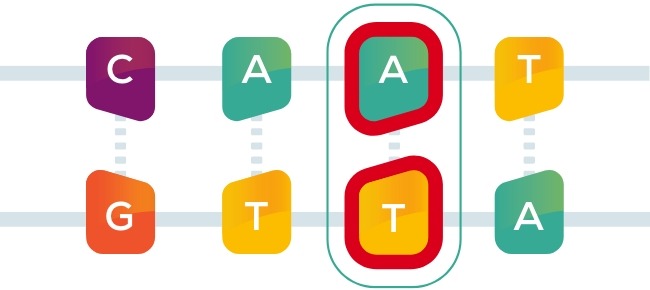
Correcting the spelling error
To correct the misspelling, we use a new tool called base editing, which works like an eraser and a pencil. By rewriting a single letter, base editors may correct disease-causing point mutations and potentially create life-long cures for patients suffering from serious diseases.
Base editing has the potential to correct a disease-causing point mutation with just a few treatments—or possibly with one treatment that may last a lifetime.
In addition to correcting single point mutations, base editing technology has multiple, highly versatile applications, including turning off genes with disease-causing activity, turning on genes to restore or increase function, changing how proteins bind or signal without disrupting their function, and targeting multiple pathways simultaneously.

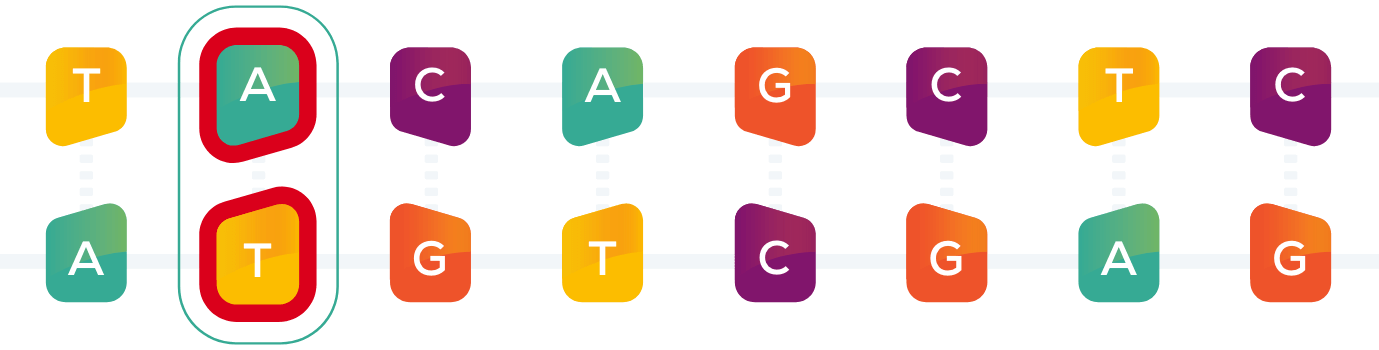
Understanding Delivery
To address the unique characteristics of each disease, Beam uses different technologies to deliver therapies to the relevant target organs.
![]()
IN VIVO
In vivo means the base editor is delivered directly into organs of the body, such as the liver, to make edits within the target cells.
We are using non-viral lipid nanoparticles (LNPs) for direct in vivo delivery to the liver in our GSDIa and alpha-1 antitrypsin deficiency programs.
![]()
EX VIVO
Ex vivo means that cells are removed from the body, edited, and then returned to the body.
Beam is using electroporation for delivery to blood cells and immune cells for ex vivo delivery in our sickle cell disease program.
Learn More
Our Science
Beam is a values-driven organization with a vision of delivering life-long cures to patients suffering from serious diseases.
Our Pipeline
We believe leveraging ex vivo and in vivo approaches, different delivery modalities, and multiple editing approaches will help us target a broad range of diseases with limited or no disease-modifying treatment options.
Our Disease Areas
We seek to potentially cure serious diseases and address significant unmet medical need so that people can reach their full potential. We believe leveraging ex vivo and in vivo approaches, different delivery modalities, and multiple editing approaches will help us target a broad range of diseases with limited or no disease-modifying treatment options.
Our Focus on Patients
 Play Video
Play Video
Sickle cell disease
Living with Sickle Cell: Kyle’s Story
 Play Video
Play Video
Alpha-1 antitrypsin deficiency
Living with AATD: Heather’s Story
 Play Video
Play Video
Glycogen storage disease Ia
Living with Glycogen Storage Disease Ia: Alyssa’s Story
Resources*
Gene Therapy 101
Available from the American Society of Gene and Cell Therapy at
https://patienteducation.asgct.org/gene-therapy-101/gene-therapy-approaches
Gene Editing
Available from the American Society of Gene and Cell Therapy at
https://patienteducation.asgct.org/gene-therapy-101/gene-editing
Understanding the Importance of Gene Therapy for Rare Diseases
Available from the National Organization for Rare Disorders at
https://rarediseases.org/gene-therapy/
Other Resources
- MedlinePlus (online health information resource from the National Library of Medicine)
- American Society of Gene + Cell Therapy
- National Organization for Rare Disorders: Gene Therapy Fact Sheet | FAQs | Gene Therapy 101 Podcast
- Global Genes: Guide to Gene Therapies | Understanding Gene Therapy Podcast
- Institute for Gene Therapies: About Gene Therapy | Understanding Value of Gene Therapies
* These resources are for information only. Inclusion of these resources does not indicate endorsement by Beam Therapeutics of an organization or its communications. The list is not intended as a comprehensive list of resources, nor are the resources intended to provide medical advice.
Our Research
An important part of the drug development process is recognizing and validating what matters to patients. To understand their needs, we collaborate with clinical experts, patient advocacy organizations, and disease communities to optimize our clinical trial design and the participant experience.

