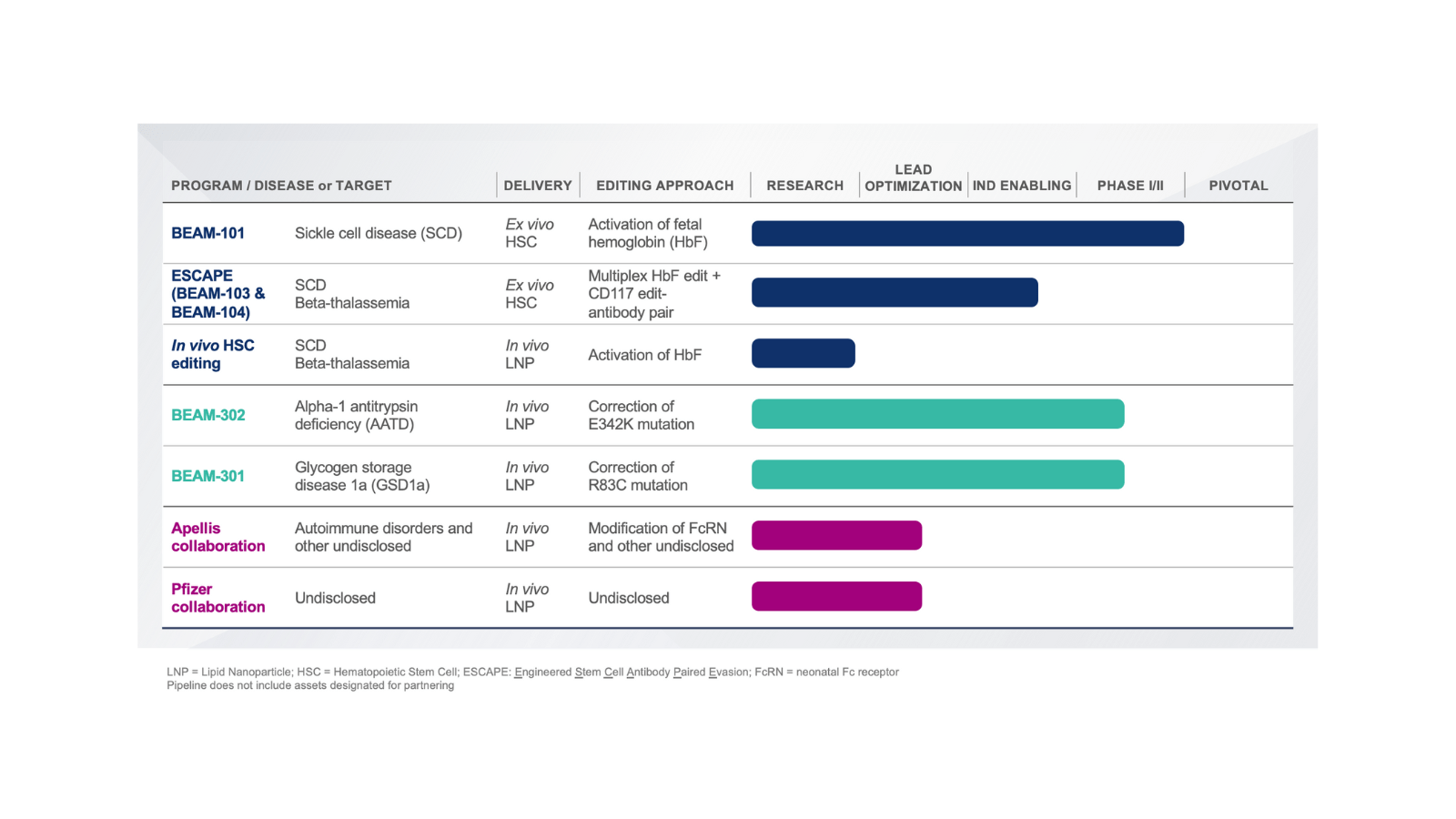
Pipeline Broad and diversified portfolio
We seek to potentially cure serious diseases and address significant unmet medical need so that people can reach their full potential. We believe leveraging ex vivo and in vivo approaches, different delivery modalities, and multiple editing approaches will help us target a broad range of diseases with limited or no disease-modifying treatment.
Scroll horizontally to see more
Lead programs Sickle cell disease
Sickle cell disease (SCD) is a group of genetic disorders that affects hemoglobin, the molecule in red blood cells that delivers oxygen to cells throughout the body. People with this disease inherit two copies of a gene, each with a single letter misspelling. This misspelling causes production of atypical hemoglobin molecules called hemoglobin S, which can distort red blood cells into a sickle, or crescent, shape, blocking the flow of blood and oxygen throughout the body. The characteristic features of SCD begin in childhood and may include anemia, repeated infections, and episodes of pain. The severity of symptoms varies from person to person and throughout life. The diminished oxygen delivery to tissues and organs can lead to life-threatening complications, such as stroke and organ damage. Chronic organ damage is the leading cause of death in adults with SCD.
Beam is advancing multiple base editing programs for sickle cell disease, led by Beam-101, an ex vivo cell therapy in which cells are collected from a patient, edited, and then infused back into the patient. To create space for the edited cells to take hold in the bone marrow, patients undergo a conditioning regimen, such as treatment with busulfan, the standard of care in hematopoietic stem cell (HSC) transplantation today.
BEAM-101
Edit type: Activation

Delivery modality: Electroporation | Ex vivo
Approach: Activation of fetal hemoglobin
BEAM-101 is an investigational therapy that produces base edits designed to potentially alleviate the effects of sickle cell disease by mimicking genetic variants seen in individuals who have hereditary persistence of fetal hemoglobin.
LONG-TERM COMMITMENT TO SCD
Today’s conditioning regimens are associated with significant toxic effects. Future improved conditioning regimens that produce fewer toxic effects could potentially be paired with BEAM-101 and with other future programs. Beam’s ESCAPE program uses base editing to enable a potentially new non-genotoxic conditioning option for patients with sickle cell disease.
Beam is also exploring the potential for in vivo base editing programs for SCD, in which base editors would be delivered to a patient through an infusion of lipid nanoparticles (LNPs) targeted to HSCs, eliminating the need for transplantation. This approach has the potential to provide a more accessible option for patients, particularly in regions where ex vivo treatment and transplantation are not widely accessible.
Alpha-1 antitrypsin deficiency
Alpha-1 antitrypsin deficiency (AATD) is a potentially life-limiting genetic disorder that may cause disease in both lung and liver function. AATD is caused by changes in the SERPINA1 gene, leading to lower levels of circulating Alpha-1 antitrypsin protein in the blood (needed to protect the lungs from damage) and the build-up of toxic mutant protein in the liver. The vast majority of AATD diagnoses are in individuals with two copies of the PiZ variant (PiZZ), a single letter misspelling in the SERPINA1 gene. People with AATD usually develop symptoms of lung disease between 20 and 50 years old. Many individuals with AATD remain undiagnosed, specifically people with obstructive pulmonary disease (COPD) or emphysema. Liver disease can develop from infancy through adulthood. The severity, signs, and symptoms of the disease as well as the age at which they appear vary among individuals even within the same family.
BEAM-302
Edit type: Gene correction

Delivery modality: LNP | In vivo
Approach: Correction of the PiZ allele
BEAM-302 is a liver-targeting LNP formulation of base editing reagents designed to correct the PiZ allele, the most common gene variant associated with severe Alpha-1 antitrypsin deficiency.
Glycogen storage disease 1a
Glycogen storage disease type 1a (GSD1a) is a serious, potentially life-threatening genetic disease with no approved disease-modifying treatments available to date. In patients who have GSD1a, glycogen accumulates in organs and tissues such as the liver, kidneys, and small intestine. The accumulation of glycogen (and sometimes fat) and inability to generate glucose during fasting can lead to severe and life-threatening hypoglycemia, seizures, metabolic dysfunction in lipids, short stature, liver adenoma, and renal disease.
BEAM-301
Edit type: Gene correction

Delivery modality: LNP | In vivo
Approach: Correction of R83C mutation
BEAM-301 is a liver-targeting LNP formulation of base editing reagents designed to correct the R83C mutation. R83C is the most common mutation responsible for causing GSD1a.

